Many pathological contexts are characterised by an abnormally acidic pH. These include sites of inflammation in autoimmune conditions, the inflamed airway in respiratory diseases and the tumor microenvironment (TME). Far from being a bystander phenomenon, this low pH signals to local immune cells through a family of acid-sensing GPCRs that are enriched on their cell surface. A key member of this family is GPR65 which has been shown to be exclusively expressed on cells of the immune system.
Click on the video below for an explanation of how GPR65 influences the immune cell types in which it is expressed and how Pathios’ molecules can reverse this signaling to achieve potentially transformative outcomes in cancer.
Whilst the advent of T-cell checkpoint inhibitors has revolutionised cancer treatment for many tumor types, it remains the case that the majority of patients have an inadequate response to these therapies. The reason for this poor response rests with the key features of the immunologically hostile tumor microenvironment (TME) that is present in many solid cancers. These features include a high angiogenesis and wound healing gene signature, a reduced expression of proinflammatory cytokines and chemokines and consequent exclusion of important effector cells, a suppression of interferon and antigen presentation genes, and a dominance by immunosuppressive tumor associated macrophages (TAMs) and other pro-tumorigenic myeloid cells.
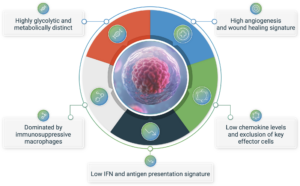
The critical features of the immunologically hostile TME that explain a lack of or poor response to currently approved immunotherapies.
In many cancers the TME becomes acidified due to the well-known metabolic switch from oxidative phosphorylation to anaerobic glycolysis and the high expression of acidifying enzymes. At the level of the local TME, low pH signals to GPR65 on a range of immune cells to cause a shift in their characteristics toward a tumor-promoting phenotype (see below). This occurs through a host of downstream transcriptional changes and can explain the critical features of the immunologically hostile TME described above. At Pathios, we are developing first-in-class drugs to suppress GPR65 signalling and therefore address the most pressing issue in cancer immunology today: delivering an immunotherapy treatment to the large number of patients who do not currently respond to approved T-cell checkpoint inhibitors.
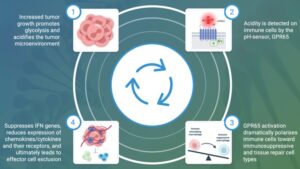
Schematic representation showing the mechanism by which GPR65 activation is the key determinant of pro-tumorigenic immune cells and therefore dictates the properties of the immunologically hostile TME.
The therapeutic potential of inhibiting the GPR65 pathway in cancer is underscored by the effect of a coding variant in the human GPR65 gene which causes a reduction in receptor signalling. Patients who are homozygous for this coding change (∼1.5% of all cancer sufferers) exhibit a significant survival advantage compared to other genotypes across multiple tumor types (see below). This survival advantage is present even in the face of highly glycolytic tumors that would be predicted to respond poorly to current T-cell checkpoint immunotherapies. These observations provide robust human genetic validation and compelling evidence that blocking GPR65 will have a significant clinical impact in a large number of cancer patients. Furthermore, inhibiting GPR65 has the potential to deliver profound monotherapy activity as evidenced by our extensive preclinical pharmacology work carried out in a range of syngeneic mouse models.
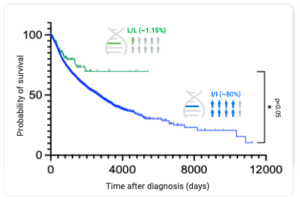
The minor allele of rs3742704 corresponds to a coding change from an isoleucine to a leucine at position 231 in GPR65 (I231L). Cancer patients that are homozygous for this variant, which leads to a reduction in receptor signalling, exhibit a significant survival advantage across multiple tumor types represented in The Cancer Genome Atlas (TCGA).
In addition to cancer, GPR65 plays a key role in a host of other immunological diseases as evidenced by a rich array of human genetic evidence. These include inflammatory and autoimmune diseases such as Inflammatory Bowel Disease and Ankylosing Spondylitis as well as CNS disorders with an immunological involvement such as Multiple Sclerosis and Parkinson’s Disease. At Pathios we have created a proprietary and extensive toolkit around GPR65 which enables us to explore the potential for small molecule GPR65 modulators in these additional diseases.
Our ground-breaking scientific approach and impressive data presented in downloadable poster presentations.